August 14, 2025: My Science Cruise Scientific Reading List
Reading on the cruise has been a challenge. While I was still getting my “sea legs” I felt too sick to make sense of a sentence. Once I got my sea legs, I was swept up into the business of the ship and the pleasure of hanging out with the fifty-six brilliant people on board, leaving little time to focus on the written word. Nonetheless, I did manage to get a few good reads in. For my last blog post I thought I could share the titles and some initial thoughts on how these papers fit in the context of my own research.
Dissolved organic carbon compounds in deep-sea hydrothermal vent fluids from the East Pacific Rise at 9°500N
- Longnecker et al., 2018
Just like my own sampling, this study began with a Jason dive to hydrothermal vents. They sampled directly from the vent fluids of hot and cool vents to find out what chemically distinct molecules are contributed by hydrothermal vents. On my first pass of this paper, I noticed that the number of compounds they looked at is relatively limited compared to another dataset I have been studying. I’m curious if this is because those compounds were the only ones present in the sample, if it was a limitation in their methodology, or if something inherent in the samples limited the scope of metabolites that could be detected. The first compromising factor that could limit my own study is pH. If the acidity of the sample disrupts the chemistry of sample processing, I may also not be able to detect quite as many compounds as are found in less extreme environments. The other observation I made in this paper is that they were focused primarily on the question of what compounds are contributed to the environment by the hydrothermal vent. Whereas my questions are more about what compounds exist within the microbial cells and separately in the “micro” environment they live in.
Molecular composition of dissolved organic matter from young organic-rich hydrothermal deep-sea sediments
- Brünjes et al., 2025
Rather than Jason, these researchers got their samples with the human operated vehicle Alvin. Figure 2 of this paper describes their metabolites as “Petroleum-like,” “Protein-like,” and “Humic-like,” which was surprising in that it did describe more specific compounds. I have the same question I did in the other paper: why? Is there something particularly challenging about doing metabolomics on hydrothermal vent samples or is this truly an open field for inquiry? What I did appreciate about this paper is that they have controls for background water. I still need to review the paper one or two more times before I can say much more about it, but it looks like a key paper to consider as I approach my own analysis.
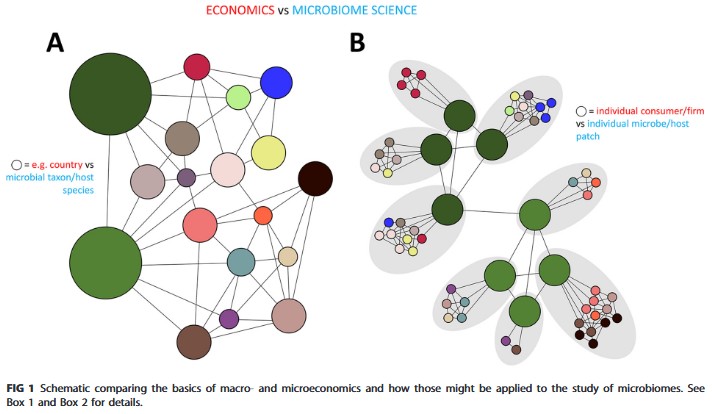
It’s the Economy, Stupid: Applying (Micro)economic Principles to Microbiome Science
- Kashtan et al., 2022
I’ve been wondering if I could apply any economic theories to explain the production and consumption of metabolites in a microbial community, and fortunately, someone else has already thought deeply about this. Figure 1 of this paper is described in economic terms in Box 1 and in microbiology terms in Box 2. The main point of this figure is to explain the difference in macro- and micro-economic perspectives, and how important limitations of microbiology could potentially be solved by shifting from a “macro-economic” to a “micro-economic” perspective in analysis. I still need to read this paper (and others!) more deeply on shore to determine if I can appropriately use economic theory in my metabolomic analysis, but my intuition says yes. I’ve collected and separated the cells of the sample from the aqueous environment they live in. There may be a way to consider the cells as producers and consumers of metabolites and use the metabolite concentrations of the water to get clues about what they are exchanging. In any case, I’m excited to read more!
August 12, 2025: A Typical Day at Sea
I’m up around 7 AM and calculate how much time I could sleep in for. Breakfast starts at 7:10 and I need to be in the Jason van by 7:45. I could use more sleep, but it’s best if I get up now. I slip into my clothes, layering up because I know it will be cold. I have never so consistently worn a beanie in my life, but it’s a regular part of my fashion on ship. I stop into the Main Lab on my way to the galley since it’s on the way. The whiteboard has a plan on it, but it’s also called the “Board of Lies” because our plans have to change so often to account for weather and how unpredictable engineering in the ocean can be. I also double check in with Mariela to see how concrete the plans are for the next few hours. With a sense of what to expect for the day, or at least the next few hours, I can start my day.
The galley is awesome. Other folks talk about what meals they skip to get more sleep, but I show up for breakfast, lunch, and dinner, and sometimes “Cheese O’Clock” too. My choice for breakfast is usually some combination of fresh fruit, some plain yogurt, oatmeal, sausages, and breakfast sandwiches. It’s hard choosing what to eat and what to leave behind. The food is good and I’m never hungry.

The rest of my morning is spent in the Jason control van. I typically sit in the Event Logger chair where I operate the Sealog system. This role consists of following along with the dive plan, paying attention to the conversations between the Science team and Jason team, and translating all of that information into a systematic log of events as they occur. This is a great job because it keeps me up close and engaged in the work being done by the ROV Jason. However, ascending and descending 2000 meters is about as fun as staring at a blue screen for two hours, which is quite literally what happens. Fortunately, the Jason team makes this more enjoyable with a good playlist and often hilarious conversation.
My shift in the control van ends right at lunch time so it’s back to the galley for me. Part of the joy of eating on ship is that someone else is cooking all of the food. If I were this busy at home, I would default to the easiest meals available to me. But on ship, I can stay busy and eat wonderful meals too. I also love that if they’re serving an unhealthy option like French fries, I can grab just a couple as a treat and keep most of my meal healthy.
After lunch and before my next shift, I have a few objectives and options. First of all, I need to work on my blog post for the day and take care of any lab work. And while I would love to knock that stuff out first, I tend to find myself exhausted by this hour and go for a nap instead. One would think that the four hours on, eight hours off shift work would lend itself to a decent amount sleep, but it is rare to have an uninterrupted eight hours of sleep. Exhaustion is pervasive throughout the ship. Shift work, exciting work, long hours, living in close quarters, and the noisy ship affect everyone. If I can muster the energy, I also try to work out during my breaks. The few times I have actually made it to the gym were great, but it should be noted that working out on the ship is bafflingly taxing. Staying steady while the boat rolls underfoot makes every exercise more difficult. At some point during my break, I typically wander back to the Main Lab and check to see how much the Board of Lies has changed. This will shape the rest of my break and usually results in me heading back up to the Jason van just to take advantage of this rare opportunity to “hang out” in the deep ocean. Soon enough, it’s time for my second shift from 8 PM to midnight. And this is, of course, after a satisfying dinner in the galley with a great homemade dessert. I’d like to say that I go straight to bed, but there are usually some cool people, cool work, and the temptation of a little bit of late-night ice cream to keep me up just a bit longer.

Life on the ship is anything but typical. We deal with varying degrees of seasickness, exhaustion, and laborious work on the deck and in the lab. We’re away from our friends and family, and there’s really not a true break when your whole world is on a 274’ ship. Despite all of this, I have only been impressed with how cheerful, collaborative, flexible, and kind everyone on board is. My guess is that we’re all on the same page in knowing that our love for the work we are doing makes it all worth the effort. Adding this community to some amazing views of the ocean, and the incredible glimpse into the deep sea, working on the R/V Atlantis is one the best experiences of my life.

August 9th, 2025: At Long Last, Hydrothermal Vent Day

Yesterday began with a wake-up call 30 minutes before the CTD cast. It was 3:30 AM. I slipped into the clothes I had already set out the night before and climbed up the stairs to the main lab to meet up with the team. The rosette was ready and before long my shallow cast was back at the surface and ready to be sampled. A few of the VISIONS students joined me in the lab where I stumbled through the first full execution of my protocol. Learning from my mistakes, we made a plan to reset the lab equipment to be more efficient for the next round of samples. I then made my way up to the Jason van for my shift.

This was the main event: hydrothermal vents! The back bench was full, everyone wanting to get the best view when Jason finally reached the ASHES hydrothermal vent field. Throughout the cruise, I have had many friends and family following along with the live stream and so I had their enthusiasm buzzing into my phone as well. I had to contain my stadium level excitement into this shipping container control van to focus on my role logging the dive. When Jason finally got to the vents it was amazing. I have seen countless videos and images of hydrothermal vents, but it doesn’t compare to what it is like to actually be involved in the expedition. The control room has a wall of monitors to show live feeds from all the cameras on the ROV. This gives you many angles to form something closer to a three-dimensional model in your mind as Jason navigates the ocean. Jason is controlled by a pilot, an engineer, and a navigator. A scientist or engineer from the RCA team directs the dive, and an event logger and video logger provide documentation and control imagery collection. All of these people were experiencing the wonder of life at 1500 meters together. I wasn’t surprised by anything I saw, I’ve read so much about hydrothermal vents, but seeing it with Jason clicked so many pieces together for me as if I was standing on the ocean floor myself.

But I didn’t come here just to look at the vents, I wanted samples! One of the highlights from my day was getting to sit in the hot seat and work with the Jason team to actually direct the ROV’s movement. I had an idea of how I wanted to sample, but was unsure of what was actually possible. All of my expectations were exceeded. The ROV pilot got Jason right next to the vent so that the Niskin, positioned on the side of the vehicle, was placed up against the communities of palm worms, tube worms, and limpits. Once the Niskins were fired, I went back down to the lab and prepare a few things to be better for this round of sample processing. When Jason surfaced, we quickly took the water, returned the Niskins ,and got to work. This time was much smoother and ended with a clean reset of the equipment so that we were prepared to start the next round. By this time, my second shift was starting. Chief Scientist Katie had already checked in with me to make sure I was doing okay having been up for so long. It was only when I sat down to start logging for Jason, did I feel myself start to hit a wall. When it was time to direct my next sample collection, this time with the pilot Akel, I had to take a breath to collect myself for this last push of effort. Once the Niskins were fired I asked one of my teammates to cover for me and went to close my eyes. Maybe an hour later, I got another wake-up call that Jason was surfacing and it was time to process these samples again. This time, everyone knew what they were doing and the entire protocol was executed perfectly. It was nearly midnight so we finished with a rinse of all the equipment and went to bed. I want to acknowledge that the VISIONS students on board were so essential to me processing these samples in any timely manner. Thank you so much Ari, Isabelle, Megan, Nadia, Nicole, and Anshul! Whether it was rinsing containers, running filter lines, taking notes, or simply covering my shift, I couldn’t have done it without you.

Science is a collaborative effort. Just to get ready I had a ton of help from my advisor, my lab mate, so many people from the Ingall’s lab. There is a constant collaboration on the ship between the science, APL and Jason teams, and the ship crew to make this mission possible. Katie and Mariela have been incredibly supportive of incorporating my opportunistic samples into the RCA goals. And again, the students who were eager to help made all this sampling possible to complete in just one day. If I’ve learned anything on this ship, it’s teamwork and I’m so privileged to be on this team doing this work.
August 7, 2025: My Science Dreams are Coming True
This blog post will be short and sweet so that I can get plenty of rest for what will be one of the most exciting days of my life tomorrow. I have wanted to sample a hydrothermal vent since I first learned about them and have taken many steps over the last five years to prepare and put myself in the position to make this happen. For me, it’s the most ecologically interesting ecosystem that has so much to teach us about what life is possible in the universe. Nothing is cooler (or should I say hotter?) than a hydrothermal vent.

The plan is to start the day at 4 AM with a CTD cast to collect samples at 40 meters depth, the chlorophyll maximum, and 5 meters “surface”. These will serve as my surface control samples. At 6 AM, Jason will dive to the ASHES hydrothermal field at the summit of Axial Seamount to turn the HD camera, install a platform and recover an osmotic fluid sampler installed near the Mushroom vent. Jason has two Niskin bottles installed on its side that can be triggered at any time. I will be in the control van to direct where these samples are taken. I need the water to have as much biological influence as possible, but Jason can only get so close to the high temperature vent before risking the integrity of the vehicle. Fortunately, the pilots (Chris H and Chris J) are awesome and excited for me as well so I’m sure we’ll be able to work together to find the best spots to get these Niskin samples.
After that dive, I will take the Niskin bottles off Jason, transfer the fluid sample to my lab as fast as possible, and return the Niskins back to the Jason team for them to dive again. While I am processing my samples, Jason will conduct another dive in ASHES to turn a cabled CTD in an area without visible hydrothermal activity. One Niskin will be used by the OOI-RCA team for instrument verification and I will take the other. This will serve as another control, but for deep background water. In the best case scenario, timing and weather will allow us to dive again to explore and sample more hydrothermal vents. But this is not something I’m counting on. We have a methane seep to visit when we transit back to the Cascadia Margin – Southern Hydrate Ridge, which should also prove to be a fascinating site for metabolomic analysis.

August 6, 2025: [Not] getting some reading done on transit day
Today around 3 AM Pacific Time, we began transit to Axial Seamount. As I write this blog post it is nearly 8 PM and we are just beginning to slow down. Folks around the ship have been calling this “Transit Day,” but it took experiencing it firsthand to realize that it’s not just a day where transit happens, but where transiting defines the mood and morale. We can’t put the ROV Jason or the CTD rosette in the water while the ship steaming ahead, leaving most of us with little to do. My plan for the day was to read some papers, summarize them, and write this blog post about that. But the motion of the boat changed everything. Simply walking around felt like a drunken stumble, never certain where the floor would be. Reading and writing felt impossible when the words on my screen moved dramatically along with the ship. And all day, I carried around a queasy feeling that made any activity uncomfortable. I wasn’t alone in this either. It seemed like most of the students (except for a lucky few) were affected by some degree of seasickness today. Even the more experienced scientists and engineers on the ship seemed to be uneasy, whether that was from seasickness or boredom I’m unsure. The only thing that truly helped me was curling up in my bunk.
Before I left shore, another graduate student in the School of Oceanography told me that sleeping on a boat is like being rocked in a cradle. I experienced this to be true. Sleeping through the waves was actually quite wonderful! I found immense relief from seasickness once I finally gave up on being upright and cognitively engaged.
Flexibility is key to working on a ship. I expected this in the form of changing from one productive plan to another, problem solving, and teamwork. I did not expect to be unable to work because of only mild seasickness. On paper, today was supposed to be an easy day with few responsibilities and a lot of time to be productive. In reality, it was an incredibly long, rough, and unproductive. Doing science at sea is inherently challenging and my experience today gave me a new respect for oceanographic field work. It’s not just the challenge of planning and preparing to do work on the ship or the element of surprise that comes with any environmental sampling, we are really at the mercy of the ocean just to be out here in the first place.
August 5th, 2025: Why Study the Metabolites of the Ocean?

Metabolites are small biologically synthesized molecules. Whether you have studied biology or not, you are probably familiar with the crucial role metabolites like hormones, amino acids (the building blocks of protein) and vitamins have in your own body. Microorganisms use metabolites to adapt to their environment, send signals to their community and other organisms, and even antagonize predators and prey. Metabolites are also considered a large pool of organic carbon that is passed around marine life and discarded into the deep ocean, making metabolites an important parameter when modelling the carbon cycle on local and global scales.
In addition to understanding the carbon cycle, metabolites can tell us a lot about ecological activity. Where DNA can tell us about what cellular processes are possible, metabolites are evidence of processes that have already occurred. Remember that not every gene in an organism’s DNA will be used during its lifetime. But when a metabolite is found, it tells us what kind of activity a cell has already performed. For example, a lot of research has been conducted on the microbially synthesized metabolite DMSP. DMSP is used as a signaling molecule for chemotaxis (triggering cell movement), plays a role in some pathogenesis, and is released as a stress response. If we know variables such as the microbial community composition or environmental conditions, detection of DMSP can confirm specific ecological activities.
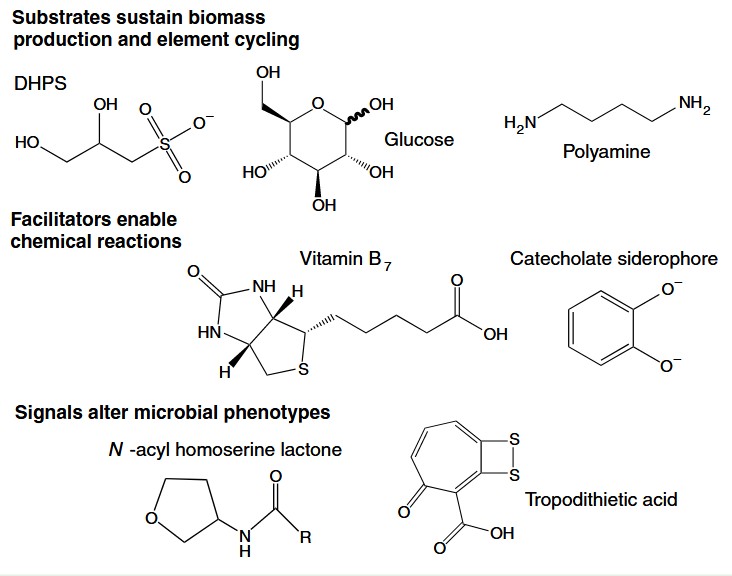 Unfortunately, metabolites are difficult to measure. Environmental samples must go through precise sample preparation and tested with mass spectrometry against known standards to determine if the target molecule is present. The Ingalls’ lab in the UW School of Oceanography has developed novel sample preparation protocols and built an extensive library of standards, which opens the door to detect a wide range of metabolites in marine samples. But their work has mostly been based on samples from the surface ocean. The samples I collect at the ASHES hydrothermal vent site will be the first of its kind to be measured in their lab and the first to be measured with the specificity their technology enables.
Unfortunately, metabolites are difficult to measure. Environmental samples must go through precise sample preparation and tested with mass spectrometry against known standards to determine if the target molecule is present. The Ingalls’ lab in the UW School of Oceanography has developed novel sample preparation protocols and built an extensive library of standards, which opens the door to detect a wide range of metabolites in marine samples. But their work has mostly been based on samples from the surface ocean. The samples I collect at the ASHES hydrothermal vent site will be the first of its kind to be measured in their lab and the first to be measured with the specificity their technology enables.
So, what do we expect to find? The first goal of my project is to determine if we can even get detectable amounts of metabolites in a Niskin sample using the ROV Jason. There’s a non-zero chance that the result of my work on VISIONS’25 is simply documenting why the collection process failed and how to improve it in the future. If we are able to detect metabolites in the hydrothermal vent samples, my first line of inquiry will be comparing it to the metabolomic profiles of the surface ocean. Many of the most abundant metabolites, such as amino acids, are ubiquitous. But hydrothermal vents are extreme environments that may change the chemistry of life needed to survive, so it is reasonable to expect these samples to be different. And if the hydrothermal vent samples are significantly different from the surface samples, it will tell us what is truly essential for life. In either case, we’ll learn more about the limits of life!
August 4, 2025: Preparing for Ocean Exploration

Planning how to conduct science on a ship was exceptionally fun. I started thinking of the type of sample collection I would want to do on the VISIONS’25 cruise a little late, only two weeks before the start of Leg 1. Fortunately, with the support of my advisor, Jodi Young, everyone from the Ingall’s Lab, and Chief Scientist Katie Bigham I felt confident I could execute metabolomic sampling of hydrothermal vents. On shore, this meant asking a ton of questions about standard protocols, figuring out what to pack, doing practice runs to ensure I would have everything I needed, and double checking that all the equipment was functional. I found that “dry runs” of the sample collection process were particularly informative. Over and over again, I would mime carrying a bin of collection bottles to retrieve water from Niskins, bringing them over to the peristaltic pumps, and running clean water through to the end. This is how I realized I needed extra tubing to transfer water from the Niskins to the collection bottles, which may seem obvious but hid in the cacophony of details I needed to work out. This is my first time sampling for metabolomics and also my first time sampling at sea so there were (and still are) many unknowns.
I challenged myself to imagine every way that my sample collection process could go wrong so that I could pack solutions too. For example, my lab group has two peristatic pumps and initially I only wanted to bring one. My concern was that if something happened to the pump at sea at least we would still have one back in the lab. But on the flip side, if something were to happen to that one peristaltic pump at sea I would have no way to continue sampling. I brought both, as well as multiples of everything else. This led to me doubling the amount of cargo I was bringing on ship which felt excessive, but once I saw what the RCA team was bringing, my set up seemed relatively simple. Conducting any science at sea is limited by what you bring, so you better bring everything.

I had prepared detailed notes on how to set up my lab bench to get started, but it proved not to be enough. One big category I underestimated was tying everything down. I had imagined that I would be unstable on a moving ship and that the big items would need to be secured, but I did not realize that EVERYTHING needs to be tied down and secured. This includes small items such as boxes of gloves, Chemtech wipes, sampling bottles, tubing and literally everything else. Writing this from the Main Lab, we even have our baskets of snacks screwed into the table to prevent them from sliding around. Once the ship left the port, I realized how important this is. I did not anticipate how much I would feel the ship rocking! The floor moves underneath our feet and I now have an appreciation for the term “sea legs.” Between the endless landscape of the ocean and the movement of the ship, it genuinely feels like we are at the mercy of the ocean. Witnessing the magnitude of the ocean first-hand has made me realize how much more there is to explore and makes me even more excited to be part of a team doing just that.
